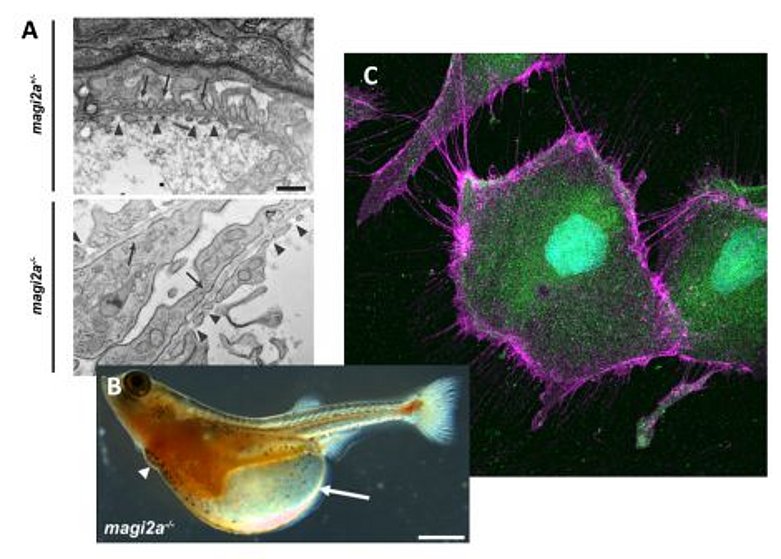
Our group is interested in the hereditary background of proteinuric kidney disease, and how genetic causes affect signaling pathways in podocytes.
Proteinuria describes the state of plasma protein loss via the urine. Proteinuric kidney diseases are divided into glomerular or non-glomerular forms, depending on whether protein loss occurs across the glomerular filtration barrier or results from impaired reabsorption of filtered protein by the proximal tubule.
Glomerular forms are caused by impairment of the glomerular epithelial cell, the podocytes, either as a primary podocytopathy or secondary to external factors. Primary podocytopathies often have a genetic background with mutations affecting the function of structural proteins of the filtration barrier or proteins involved in regulation of the actin cytoskeleton. Secondary causes (that may have a genetic background as well) include metabolic conditions like diabetes mellitus or adipositas, and dysfunction of the immune system.
In an internal cooperation with the group of Prof. Michael Wiesener and the Institute of Human Genetics we are employing Whole Exome Sequencing to identify underlying genetic causes in our local patients with proteinuric kidney disease, and employ patient derived primary cells to analyze the detrimental effects of the candidate variants identified.
In this context, we are focusing primarily on the Wnt/β-catenin pathway, a signaling pathway that is important in development, but which is reactivated in podocytes and tubular cells upon cellular stress (such as proteinuria) as a protective mechanism. However, maintained Wnt/β-catenin activation is detrimental to kidney function, and drives cellular dedifferentiation with progression to chronic kidney disease. We aim to understand the underlying mechanisms and how we can modify these to alleviate disease progression. For this purpose, we are employing cell culture models, as well as zebrafish and mouse animal models.
Group leader
Scientist
Technicians
Cathiana Kolb
Susanne Rößler
Izabela Swierzy
Doctoral students
Jan Haak
Emmanuel Nedoschill
Lena Pollinger
Felix Rahe
Louis Rhode
Cooperations
- Prof. Kerstin Amann/Prof. Christoph Daniel, Nephropathologische Abteilung, Universitätsklinikum Erlangen
- Prof. Felix Engel, Nephropathologische Abteilung, Universitätsklinikum Erlangen
- Prof. Wiebke Herzog, Lehrstuhl für Entwicklungsbiologie, Friedrich-Alexander-Universität Erlangen-Nürnberg
- SFB 1350, Universität Regensburg
- Prof. Rikke Nielsen, Aarhus University, Aarhus, Dänemark
- Prof. Friedhelm Hildebrandt, Boston Childrens Hospital/Harvard Medical School, Boston, USA
- Prof. Katsuhiko Nishimori, Fukushima Medical University, Fukushima, Japan
- Prof. Katsuhiko Asanuma, Chiba University Graduate School of Medicine, Chiba, Japan